Swiss drugmaker Novartis has received US approval for its spinal muscular atrophy gene therapy Zolgensma – pricing the one-time treatment at a record $2.125m.
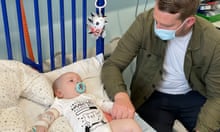
The Food and Drug Administration on Friday approved Zolgensma for children under the age of two with SMA, including those not yet showing symptoms. The approval covers babies with the deadliest form of the inherited disease as well as those with types where debilitating symptoms may set in later.
SMA is the leading genetic cause of death in infants. The disease often leads to paralysis, breathing difficulty and death within months for babies born with the most serious type 1 form. SMA affects about one in every 10,000 live births, with 50% to 70% having type 1.
“This is potentially a new standard of care for babies with the most serious form of SMA,” said Dr Emmanuelle Tiongson, a Los Angeles paediatric neurologist who has provided Zolgensma to patients under an expanded access program.
Novartis executives have defended the price, saying a one-time treatment is more valuable than expensive long-term treatments that cost several hundred thousand dollars a year.
The therapy uses a virus to provide a normal copy of the SMN1 gene to babies born with a defective gene and is delivered by infusion.
A review in April by the independent Institute for Clinical and Economic Review (Icer), concluded Novartis’s previous $5m-per-patient value estimate for Zolgensma was excessive.
But on Friday, Icer said that based on Novartis’s additional clinical data, the broad FDA label and its launch price, it believed the drug fell within the upper bound of its range for cost-effectiveness.
Novartis said it has so far treated more than 150 patients with Zolgensma. Its chief executive, Vas Narasimhan, described Zolgensma as a near-cure for SMA if delivered soon after birth. But data proving its durability extends to only about five years.
Novartis is expecting European and Japanese approval later this year. Zolgensma will compete with Biogen’s Spinraza, the first approved treatment for SMA.
Spinraza, approved in late 2016, requires infusion into the spinal canal every four months. Its list price of $750,000 for the initial year and $375,000 annually thereafter was also deemed excessive by Icer.
Wall Street analysts have forecast Zolgensma sales of $2bn by 2022, according to a Refinitiv survey. Spinraza sales hit $1.7bn last year, and are predicted to rise to $2.2bn in 2022.